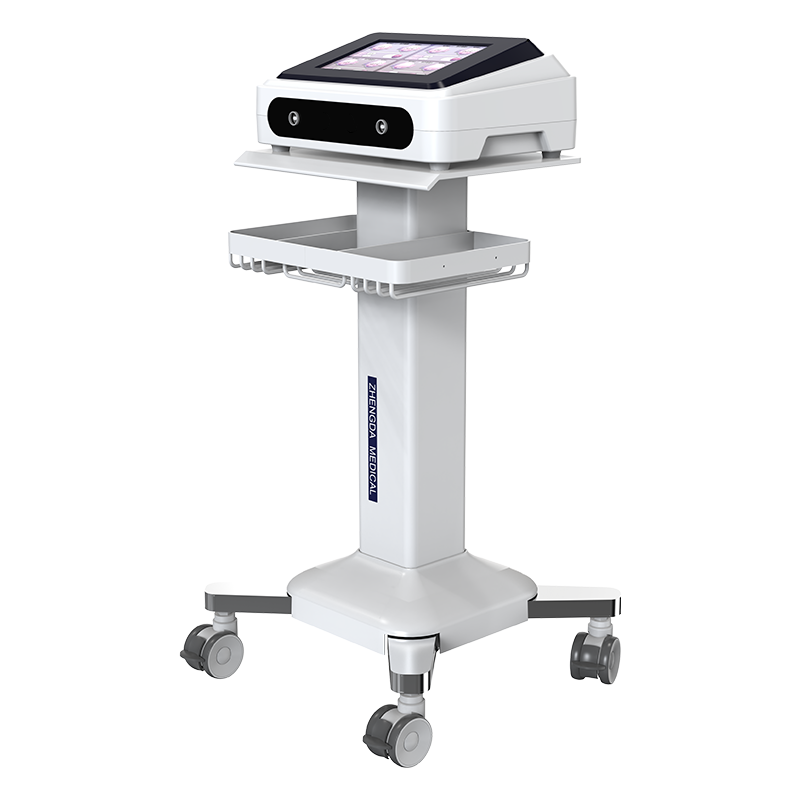
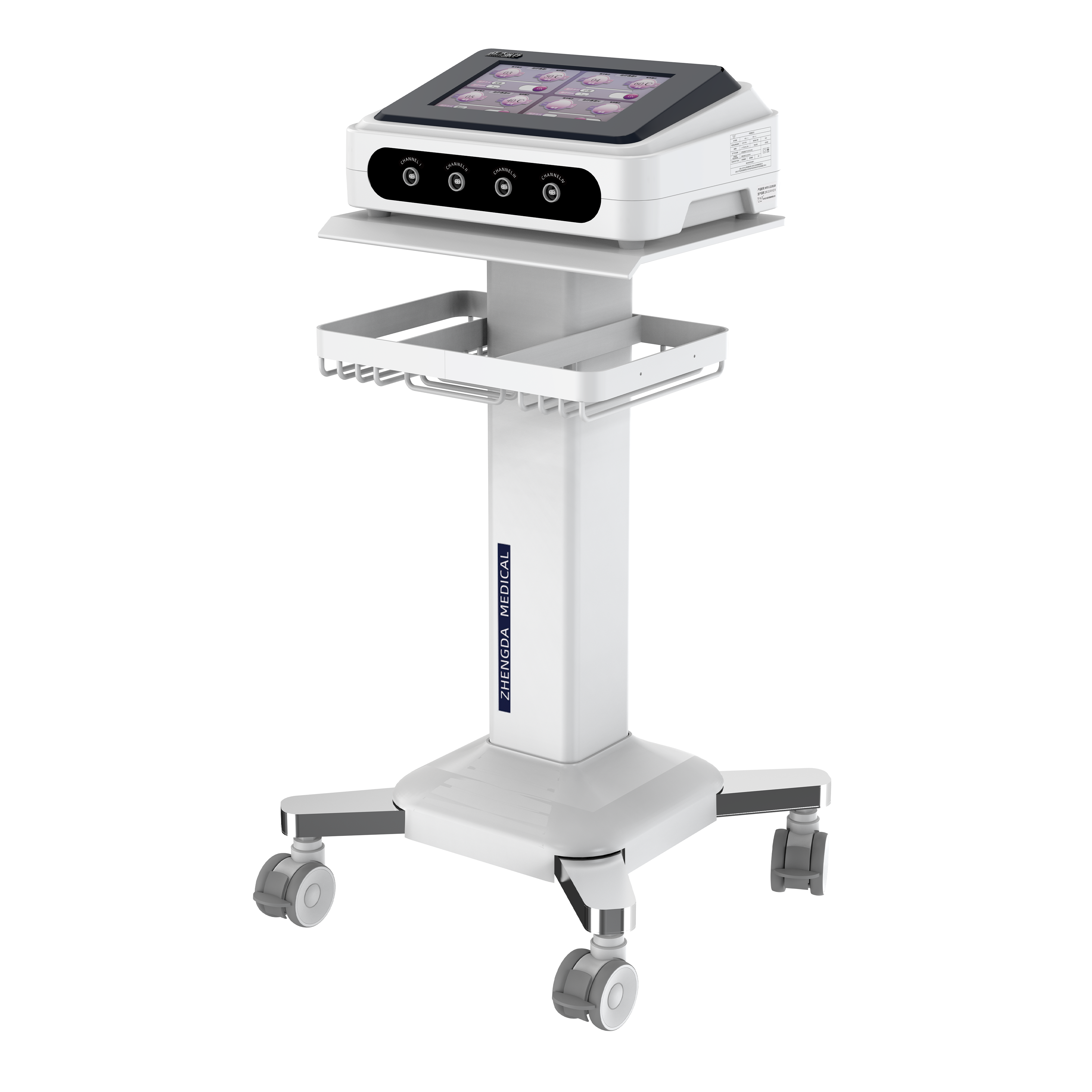
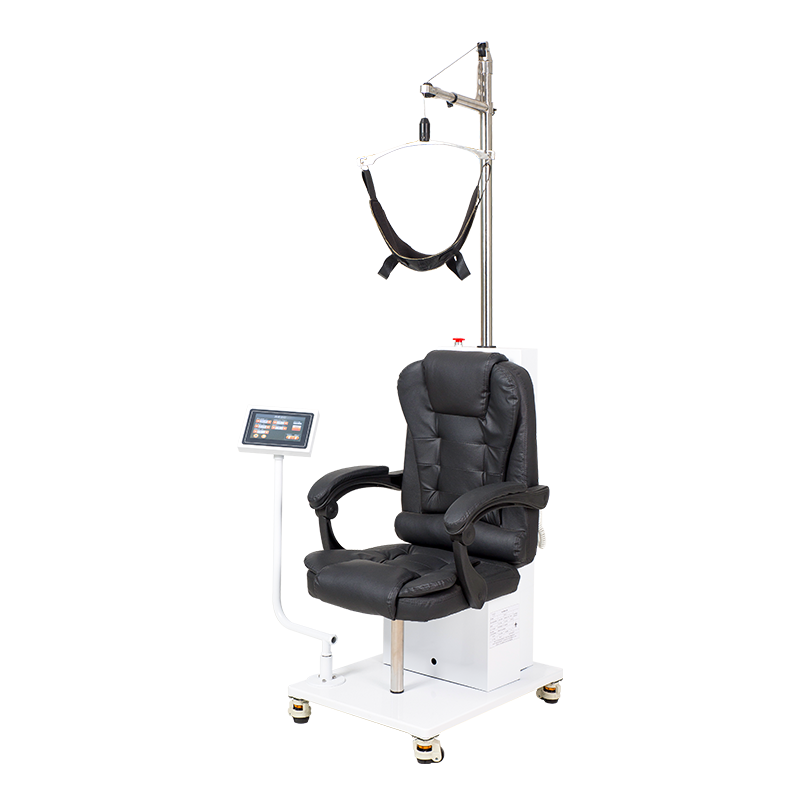
Technique parameter:
1. Effect capacity: ≥100kg
2. Supporter surface angle: -15°~75°
3. Static pressure bearing capacity of adjustable chest pad: ≥ 980N
4. Static pressure bearing capacity of head support: ≥ 98N
5. Maximum support length of adjustable chest pad: ≥ 700mm
6. Support width of adjustable chest pad: ≥ 100mm
7. Minimum adjustable distance of adjustable chest cushion support: ≤ 70mm
8. Maximum adjustable distance of adjustable chest cushion support: ≥ 160mm


 English
English Español
Español

















 Request A Quote
Request A Quote